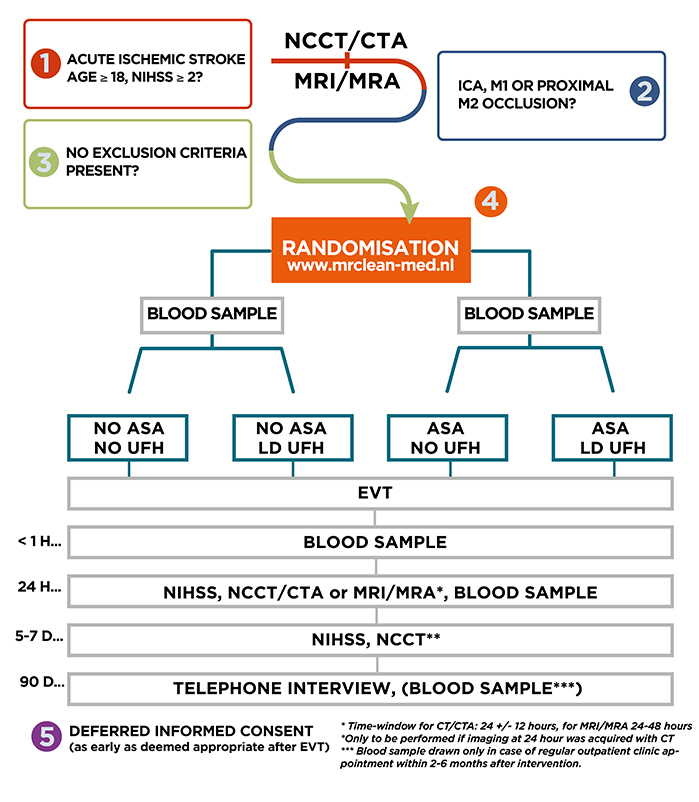
Study design
MR CLEAN-MED is a multicenter phase III randomized clinical trial with open-label treatment and blinded outcome assessment (PROBE), with a 2x2 factorial design. The study will run for 4 years in stroke intervention centers in the Netherlands.
Intervention (2x2 factorial design):
Acetylsalicylic acid (ASA):
- No administration of ASA.
- Treatment with intravenous ASA 300 mg.
Unfractionated heparin (UFH):
- No administration of UFH.
- Low dose intravenous UFH: Loading dose of 5000 IU, followed by continuous infusion of 500 IU/hour over 6 hours.